Percutaneous Ablation of Colorectal Liver Metastases (CRLM)
Background
- Incidence of colorectal cancer is 1.8 million patients and mortality is 881’000 patients per year1
- Approximately 30% of patients with colorectal cancer develop Colorectal Liver Metastasis (CRLM, up to 1.3 million patients per year)2
Indications for Ablation
- Unresectable lesions
- Combination therapy with hepatic resection
- Impaired general health status
- Small solitary lesions which otherwise require a major hepatectomy
- Patient preference of ablation over resection
Ablation Technique
- MWA or RFA3
- CT-based treatment and margin assessment.
- Margin ≥ 10 mm (Local Tumour Progession of 0%)4
- Track ablation mandatory
Guidelines
- European Society for Medical Oncology (ESMO): Ablation for tumours < 4 cm if surgery is contraindicated5 and as a first line treatment with oligometastatic disease
- National Institute for Health and Care Excellence (NICE): Current evidence adequate to support ablation for patients unfit or unsuitable for resection6
- National Comprehensive Cancer Network (NCCN): Ablation for cases that may not be optimal for resection, discussion on small solitary metastasis ongoing7
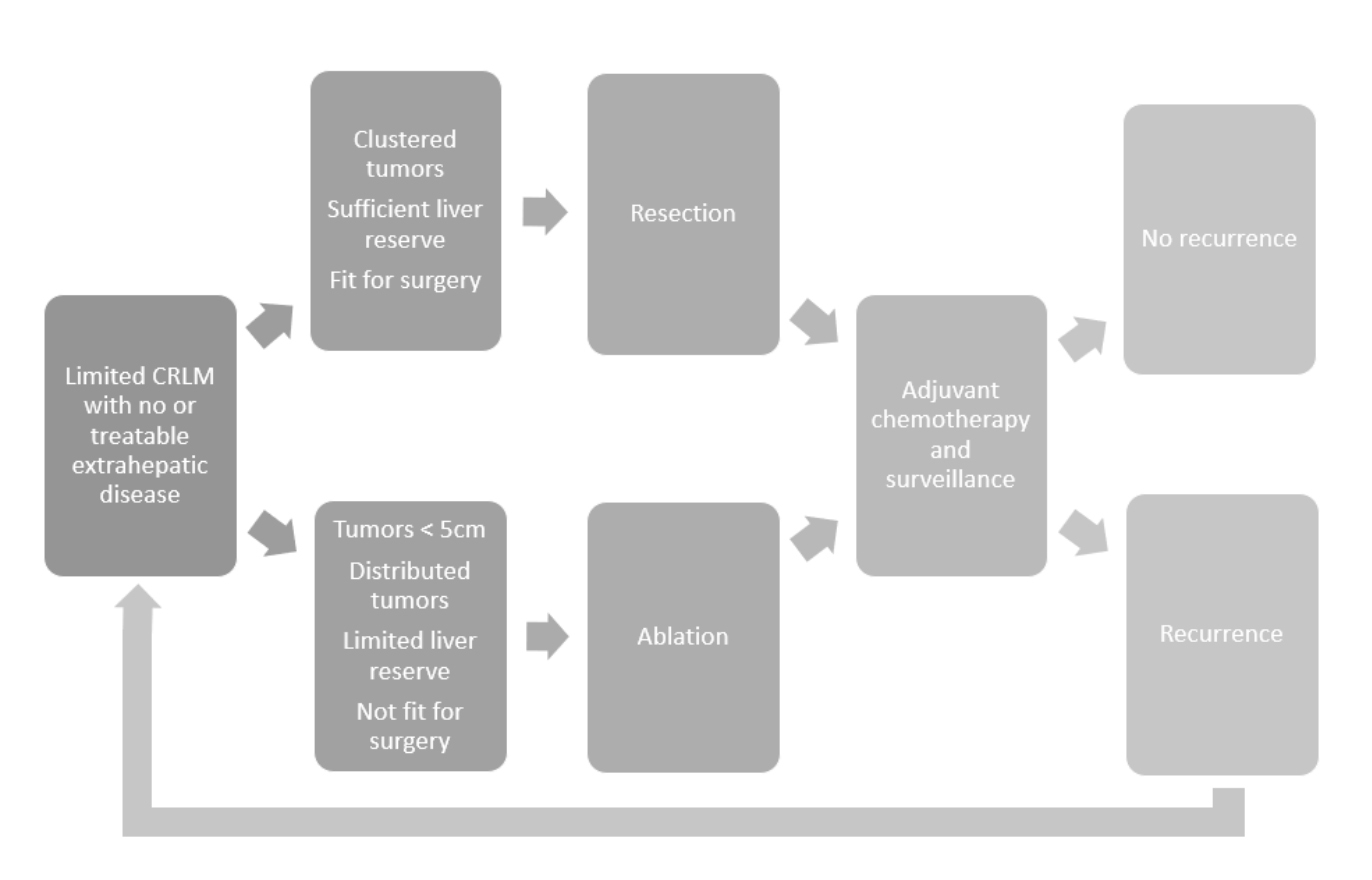
Management pathway for patients presenting CRLM from Gillama, A. et al.3
Stereotactic CRLM Ablation with CAS-One IR
- Shorten ablation learning curve and ensure reliable tumour destruction (97% technical success rate)15,16
- Enable access to lesions located in all liver segments through navigated off-plane trajectories17
- Verification of sufficient margin by performing a quality control of ablation during the interventional treatment and immediately applying re-ablation if required4,16–18
Relevant Studies
Ablation for non-resectable CRLM
- Improve overall survival (OS) in comparison to chemotherapy alone (8-year OS 35,9% vs. 8.9%)8
- Improve progression-free survival (PFS) in comparison to chemotherapy alone (3-year PFS 27,6% vs. 10.6%)8
- Reduce tumour load and potentially convert patients to surgically resectable3
- Avoid 2-stage hepatectomy and achieve a similar outcome with morbidity and mortality9
- Parenchyma-sparing treatment of small lesions
- Increase surgical treatability in case of multi-lobar disease3
- Higher LTP for ablation + surgery than surgery alone but no difference in OS10
- Avoid hepatectomy if ablation with sufficient margins (10 mm) are feasible
- Similar oncological outcomes to surgery11
- Reduce treatment costs compared to resection12
- MAVERRIC Trial: Non-inferiority of ablation vs. surgery for resectable tumors (up 5 lesions ≤ 30 mm)13
- COLLISION Trial: Non-inferiority of ablation vs. surgery for resectable tumors (at least one lesion ≤ 30 mm)14
References
1. Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 68, 394–424 (2018).
2. Engstrand, J. et al. BMC Cancer. 2018; 18: 78. Published online 2018 Jan 15. doi: 10.1186/s12885-017-3925-x
3. Gillams, A. et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the interventional oncology sans frontières meeting 2013. Eur. Radiol. 25, 3438–3454 (2015).
4. Shady, W. et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J. Vasc. Interv. Radiol. 29, 268-275.e1 (2018).
5. Van Cutsem, E. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27, 1386–1422 (2016).
6. Bromham, N., Kallioinen, M., Hoskin, P. & Davies, R. J. Colorectal cancer: Summary of NICE guidance. BMJ 368, m461 (2020).
7. Messersmith, W. A. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J. Natl. Compr. Canc. Netw. 17, 599–601 (2019).
8. Ruers, T. et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J. Natl. Cancer Inst. 109, (2017).
9. Philips, P. et al. Single-stage resection and microwave ablation for bilobar colorectal liver metastases. Br. J. Surg. 103, 1048–1054 (2016).
10. Imai, K. et al. Long-term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. Br. J. Surg. 104, 570–579 (2017).
11. Solbiati, L. et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: Local response rate and long-term survival with up to 10-year follow-up. Radiology 265, 958–968 (2012).
12. Takahashi, H. et al. A Comparison of the Initial Cost Associated with Resection Versus Laparoscopic Radiofrequency Ablation of Small Solitary Colorectal Liver Metastasis. Surg. Laparosc. Endosc. Percutaneous Tech. 28, 371–374 (2018).
13. Freedman, J. Microwave ablation of resectable colorectal liver metastases - one year into the maverric study. HPB 21, S901 (2019).
14. Puijk, R. S. et al. Colorectal liver metastases: Surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer 18, 821 (2018).
15. Widmann, G., Schullian, P., Haidu, M. & Bale, R. Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc. Intervent. Radiol. 35, 570–80 (2012).
16. Beermann, M. et al. 1000 consecutive ablation sessions in the era of computer assisted image guidance – Lessons learned. Eur. J. Radiol. Open 6, 1–8 (2019).
17. Lachenmayer, A. et al. Stereotactic Image‐Guided Microwave Ablation of Hepatocellular Carcinoma using a computer‐assisted navigation system. Liver Int. liv.14187 (2019) doi:10.1111/liv.14187.
18. Bale, R. et al. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur. Radiol. 22, 930–7 (2012).
2. Engstrand, J. et al. BMC Cancer. 2018; 18: 78. Published online 2018 Jan 15. doi: 10.1186/s12885-017-3925-x
3. Gillams, A. et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the interventional oncology sans frontières meeting 2013. Eur. Radiol. 25, 3438–3454 (2015).
4. Shady, W. et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J. Vasc. Interv. Radiol. 29, 268-275.e1 (2018).
5. Van Cutsem, E. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27, 1386–1422 (2016).
6. Bromham, N., Kallioinen, M., Hoskin, P. & Davies, R. J. Colorectal cancer: Summary of NICE guidance. BMJ 368, m461 (2020).
7. Messersmith, W. A. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J. Natl. Compr. Canc. Netw. 17, 599–601 (2019).
8. Ruers, T. et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J. Natl. Cancer Inst. 109, (2017).
9. Philips, P. et al. Single-stage resection and microwave ablation for bilobar colorectal liver metastases. Br. J. Surg. 103, 1048–1054 (2016).
10. Imai, K. et al. Long-term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. Br. J. Surg. 104, 570–579 (2017).
11. Solbiati, L. et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: Local response rate and long-term survival with up to 10-year follow-up. Radiology 265, 958–968 (2012).
12. Takahashi, H. et al. A Comparison of the Initial Cost Associated with Resection Versus Laparoscopic Radiofrequency Ablation of Small Solitary Colorectal Liver Metastasis. Surg. Laparosc. Endosc. Percutaneous Tech. 28, 371–374 (2018).
13. Freedman, J. Microwave ablation of resectable colorectal liver metastases - one year into the maverric study. HPB 21, S901 (2019).
14. Puijk, R. S. et al. Colorectal liver metastases: Surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer 18, 821 (2018).
15. Widmann, G., Schullian, P., Haidu, M. & Bale, R. Stereotactic radiofrequency ablation (SRFA) of liver lesions: technique effectiveness, safety, and interoperator performance. Cardiovasc. Intervent. Radiol. 35, 570–80 (2012).
16. Beermann, M. et al. 1000 consecutive ablation sessions in the era of computer assisted image guidance – Lessons learned. Eur. J. Radiol. Open 6, 1–8 (2019).
17. Lachenmayer, A. et al. Stereotactic Image‐Guided Microwave Ablation of Hepatocellular Carcinoma using a computer‐assisted navigation system. Liver Int. liv.14187 (2019) doi:10.1111/liv.14187.
18. Bale, R. et al. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur. Radiol. 22, 930–7 (2012).

